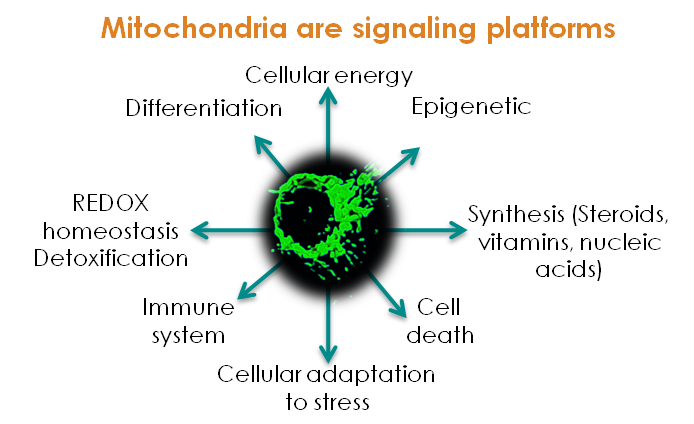
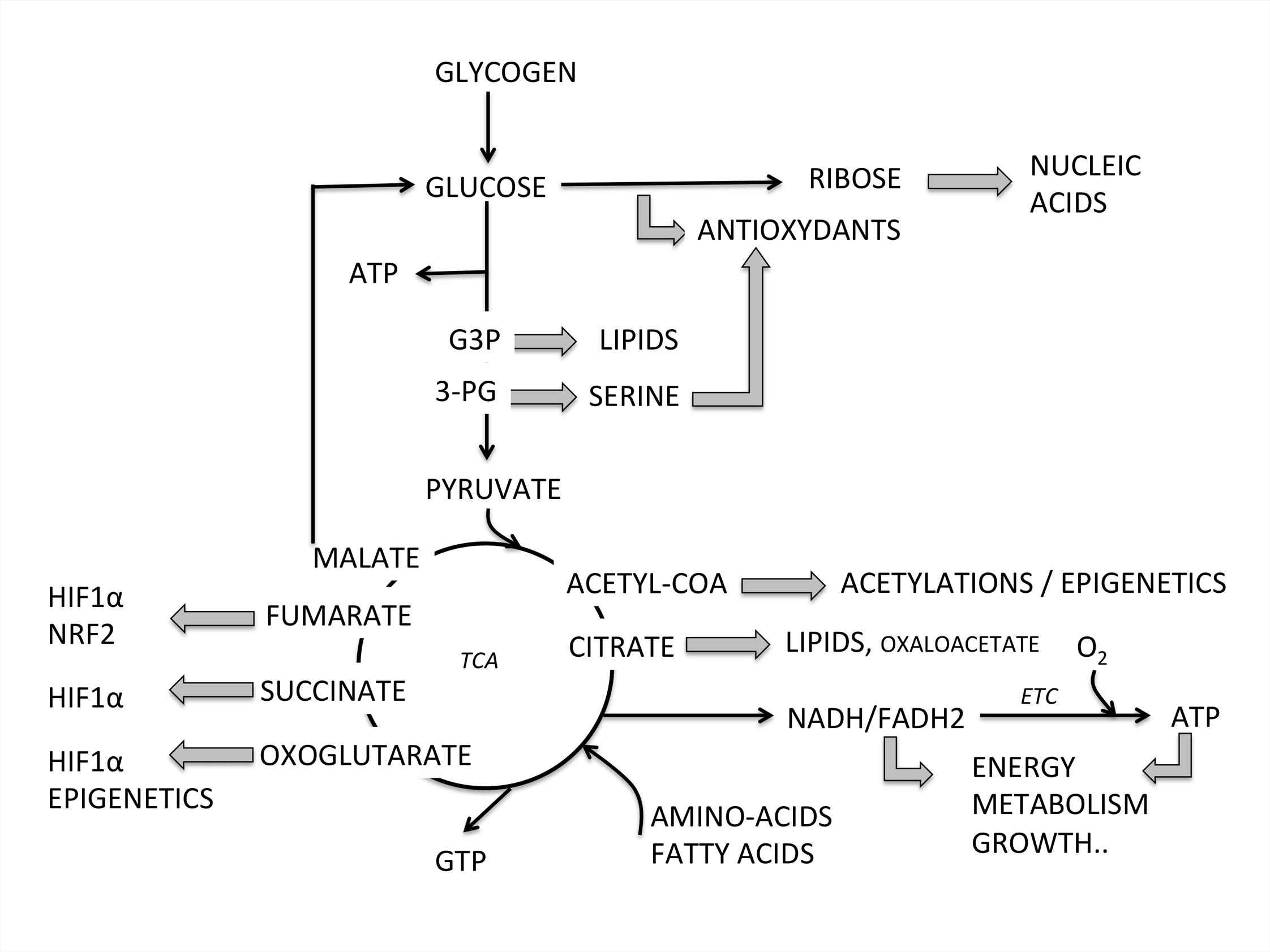
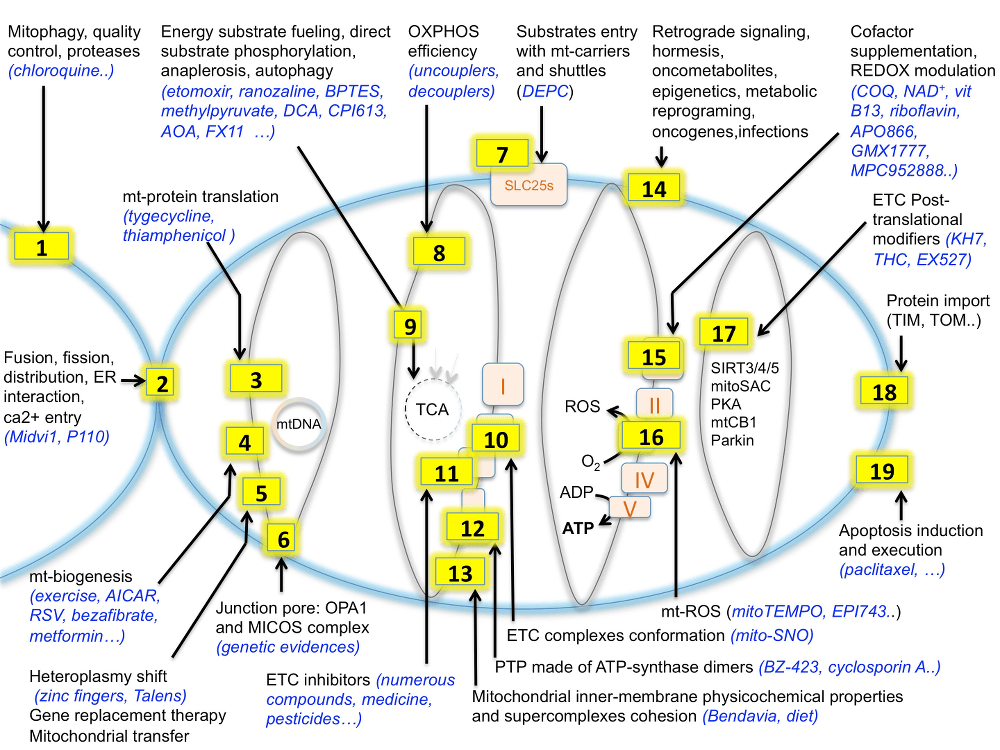
Energy metabolism is the process of generating energy (ATP) from nutrients such as carohydrates, fat or proteins, through a series of interconnected biochemical pathways. Those processes can be altered in a large number of genetic diseases or as a consequence of toxic insults.
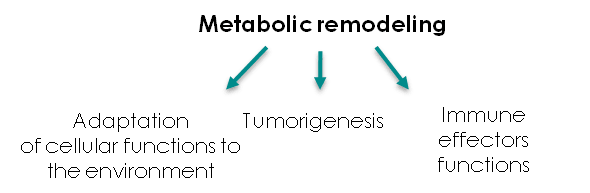
Therefore, in addition to the approximately 200 rare diseases with an alteration of energy metabolism (Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N Engl J Med 2012;366(12):1132–41), a huge number of individuals suffering from chronic illnesses and age-related diseases also present various aspects of bioenergetic alterations and could benefit from adapted bioenergetic modulation therapy (BIOMET). For instance, dysfunction and deregulation of numerous mitochondrial and cellular bioenergetic pathways (glycolysis, Krebs cycle, β-oxidation, amino-acid-degradation, energy sensing..) were reported in type 2 diabetes and chronic kidney diseases (CKD), metabolic syndrome, acute coronary syndrome (ACS), common neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s diseases, autoimmune disorders, age-related macular degeneration, optical atrophies, bipolar disorder, infertility, fatigue and several types of human cancer.
Moreover, environmental insults can alter energy metabolism, as iatrogeny, water pollution, pesticides, viral or bacterial infections and lifestyle. The aging process itself is characterized by a reduction of energy metabolism in several tissues, so that the number of individuals that could benefit from adapted BIOMET is in fact dramatically important. For references please read: Energy metabolism disorders. (Rossignol R. Int J Biochem Cell Biol. 2015 Jan 14)
- We provide expert consultancy on your problematics around energy metabolism
- We evaluate energy metabolism activity and alterations in your disease or conditions of interest
- We characterize the underlying biochemical, signaling and genetic regulatory mechanisms
- We suggest adapted bioenergetic strategies for energetic recovery or inhibition
- We evaluate the effect of adapted bioenergetic modulation strategies in your context